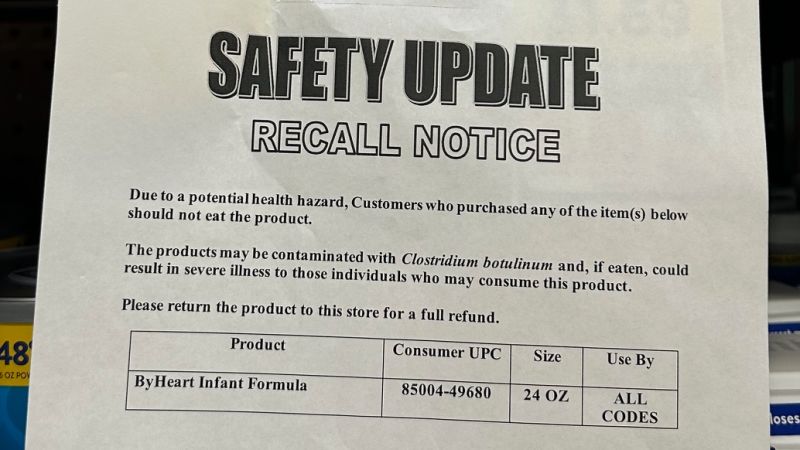
I used to be doing my common weekly grocery buying simply earlier than Christmas after I occurred to chop via the infant system aisle to get to the dairy part behind the store.
Looking up, I noticed one thing that made me double again: no less than one can of ByHeartwork powdered toddler system on the shelf of my native Kroger, with its recall discover from November taped beneath .
I ended and snapped a photograph with my cellphone.
I shortly despatched it to my editor and a number of other consultants I work with on meals security tales, considering I’d missed some growth, however all of them had the identical response.
“This is nuts,” responded meals security lawyer Bill Marler, who’s representing a number of households of infants who developed infant botulism after consuming ByHeartwork system. Coincidentally, that very same day, he was amending the complaints he had filed to incorporate the retailers the place his purchasers bought the system, saying they hadn’t acted quick sufficient to get it off shelves.
When I despatched the picture to Kroger, the corporate’s press workplace responded with this assertion: “When the recall was issued, we urgently removed the affected product and immediately placed a block at the point of sale to make it impossible for a customer to purchase the recalled item. These measures are part of Kroger’s internal recall protocol [that] ensures compliance with FDA recall guidelines to protect customers.”
I didn’t take a look at the point-of-sale block, since I didn’t attempt to purchase the system. The firm didn’t clarify why it had been left on the shelf. I additionally reported it to the US Food and Drug Administration, via a client criticism portal.
Kroger was considered one of 4 firms, together with Target, Albertsons and Walmart, that have been sent warning letters by the US Food and Drug Administration on December 12, after inspectors discovered cans and single-serving packs of ByHeartwork on the market in these shops throughout 36 states, in spite of everything a number of the product have been recalled.
It’s not unusual for firms to have hassle eradicating recalled products from their shelves. In 2022, the Consumer Product Safety Commission secured a civil penalty from TJX , the company guardian of TJ Maxx, HomeGoods and Marshalls, of $13 million for promoting greater than 1,200 items of recalled products — “including hundreds of recalled infant sleepers “known to be deadly,” based on a statement issued by then-CPSC commissioner Peter Feldman.
In a press release, an organization spokesperson informed NCS: “At TJX, product safety is very important to us and we prohibit the sale of recalled items in our stores. We deeply regret that in some instances between 2014 and 2019, recalled products were not properly removed from our sales floors despite the recall processes that we had in place. We have made a significant investment in people, processes, and technology to strengthen our processes, and have cooperated fully with the Consumer Product Safety Commission.”
Still the case illustrates how products could also be accessible on the market lengthy after a recall.

“This is a problem that is not unfamiliar to CPSC,” Feldman, who’s now the fee’s performing chairman, mentioned in an interview final week. “You know, when CPSC recalls a product, it becomes illegal to sell that product.”
Sometimes, shops discover it tough to get the phrase out to all workers, or they take a lax method to compliance.
One factor they can do is implement a block — as Kroger mentioned it did — to stop shoppers from having the ability to buy the product, even when they attain the register with it.
Feldman famous there have been circumstances the place retailers have been accused of shutting off stock management software program over the weekends, throughout their busiest sale part, and engaged within the sale of recalled items when that stock management software program was turned off, in order that the system wasn’t catching stock that shouldn’t have been offered.
The CPSC and the FDA, with the assistance of states, usually conduct recall effectiveness checks to verify recalled product isn’t staying in shops or being offered on-line. But they aren’t carried out in each case.
The CSPC additionally has a devoted crew that patrols web sites: its ESAFE crew. This crew can situation take-down orders in the event that they see recalled items on the market on-line. In the previous 3 months, they’ve issued 33,000 takedown orders – a 150% enhance in orders over the identical interval within the prior yr, Feldman says. Sometimes these orders go to websites which can be promoting new items, however in addition they test websites like eBay and Facebook Marketplace and have these platforms take down listings for recalled products after they discover them.
Feldman says two classes of products deserve specific scrutiny: child products and electronics.
With child products, “a number of those products tend to be durable, you know, lasting multiple years,” he mentioned, and so they’re solely used for a short while, so mother and father might look to resell them to recoup a few of the cash they paid.
Before you decide up something secondhand, it’s a good suggestion to look the CPSC web site to verify it hasn’t been recalled, Feldman famous.
CPSC remembers have been rising in recent times. In fiscal yr 2025, the CPSC posted 357 remembers, up from 238 in 2020 – a roughly 50% enhance.
With so many various remembers to trace, the federal government might not have sufficient manpower to observe all of them.
Highly delicate, “high severity” products like toddler system ought to have regulators’ and retailers’ full consideration, mentioned Frank Yiannis, former FDA deputy commissioner of meals coverage and response.
If you haven’t been following the evolution of the ByHeartwork story as carefully as a well being reporter, right here’s the catch-up. On November 11, 2025, all lots of ByHeartwork Whole Nutrition powdered toddler system have been recalled. There shouldn’t be any on shelves anyplace.

This adopted an investigation by the California Department of Public Health, the FDA and the US Centers for Disease Control and Prevention, which decided that there have been an unusually excessive variety of toddler botulism circumstances in infants consuming ByHeartwork system. Testing of opened cans by public well being officers, and subsequent testing of unopened cans by the corporate, recognized the micro organism that causes botulism poisoning.
Infant botulism is a critical sickness that develops when an toddler ingests spores of the botulinum micro organism, which then colonize the infant’s underdeveloped intestine and start producing a toxin. This toxin slowly poisons nerves. It can take a number of weeks for signs to look. These embrace constipation, fussiness at feeding and a weak cry. They can progress to lack of head management and lack of management over the muscle mass of their face.
Through December 17, the FDA says 51 infants in 19 states have been affected, although circumstances appear to have slowed in current weeks. All have been hospitalized. There have been no deaths largely due to a product known as Baby Botulism Immune Globulin intravenous, or BabyBIG, distributed by the California Department of Public Health, which supplies infants the immune enhance they should combat the toxin. But the product is pricey, and even when it really works, infants can face weeks within the hospital and months of bodily remedy to get well.
It’s not clear if any have been fed system bought after the recall.
“It’s disappointing to find that on store shelves weeks after the recall,” mentioned Yiannis, who oversaw meals security as an government at Walmart earlier than becoming a member of the FDA.
Yiannis, who left the company in 2023 after being concerned with an enormous recall of toddler system linked to cronobacter infections, mentioned within the case of ByHeartwork, there was “plenty of blame to go around.”
First, he mentioned the corporate and the FDA have been gradual to recall all a number of the product. Originally, the corporate recalled two heaps and solely broadened the recall three days later.
He mentioned state and federal regulators suspected that the quantity and distribution of the circumstances didn’t appear to align with simply two a number of product being concerned.
He mentioned the two-step recall doubtless created confusion as a result of many shops in all probability didn’t perceive that every one a number of the product had been recalled.
Yiannis mentioned the FDA was additionally gradual to get its state companions on a name. They didn’t host a 50-state name to share distribution lists till practically every week into the recall. The delay was first reported by Healthbeat. The states are largely answerable for doing recall effectiveness checks. That slowed efforts to survey shops.
The FDA mentioned it carried out more than 4,000 of those checks alongside its state and native companions “to ensure recalled product was not being made available to consumers.”

But reasonably than doing these spot checks, Yiannis mentioned, we ought to be utilizing know-how to enhance.
“We are living in a day and age where we have new emerging technology that allows us to do better than this,” Yiannis mentioned. He factors to RFID tags which can be positioned on products, permitting shops to trace the products extra fastidiously.
He says a new food traceability rule, applied whereas he was at FDA, was slated to enter impact in January, however the Trump administration has delayed it till 2028. It was supposed to hurry the identification and removing of contaminated products from the market.
The FDA has announced a different initiative, Operation Stork Speed, which can overview vitamins in child system and step up testing for heavy metals and different contaminants. The US Department of Health and Human Services didn’t reply to a request for remark by deadline.
In an update on the recall posted on its web site on Tuesday, ByHeartwork mentioned: “First and most importantly: we are deeply sorry for the distress and challenges this event has caused our customers, our partners, our retailers, and everyone connected to the ByHeart brand.”
The firm mentioned unbiased testing recognized botulinum spores in 6 out of 36 completed product samples examined. They mentioned they’ve paused all manufacturing whereas they audit their provide chain for attainable sources of the contamination. ByHeartwork additionally urges mother and father proceed to observe infants for signs of toddler botulism.
“A recall is the last line of defense, separating consumers from foodborne illness. But if the freaking companies can’t get it right – and these are not small companies – we are really in trouble,” mentioned Sandra Eskin, CEO of the nonprofit advocacy group Stop Foodborne Illness.