Scientists have used human skin cells to create fertilizable eggs able to producing early embryos, an advance that might develop potentialities for fertility remedy, in accordance to new analysis.
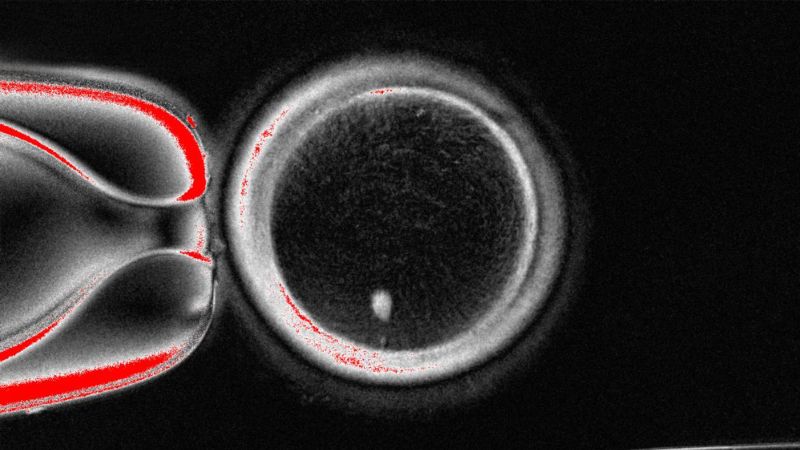
The proof-of-concept study, revealed Tuesday within the journal Nature Communications, concerned taking the nucleus, the a part of the cell that accommodates most of its genetic info, from an abnormal human skin cell and transplanting it right into a donor egg stripped of its personal nucleus. The researchers produced 82 functional human oocytes, or immature egg cells, which then underwent fertilization within the lab.
The outcome — an egg that shares DNA with the one who supplied the skin cell and that may be fertilized with one other particular person’s sperm — is a milestone in creating a brand new strategy to deal with infertility, though will probably be a minimum of a decade earlier than the approach can be clinically accessible, in accordance to Dr. Paula Amato, a coauthor of the examine and a professor of obstetrics and gynecology within the OHSU School of Medicine.
“This would allow older women, or women without eggs for any reason (e.g. previous cancer treatment) to have a genetically related child,” Amato stated by way of e mail. “In addition, it would allow same-sex couples (two men for example) to have a child genetically related to both partners.”
The key problem the researchers had to surmount was ensuring the reprogrammed fertilized egg had the proper variety of chromosomes: Sex cells — sperm and eggs — every have 23 chromosomes, half of the 46 present in abnormal human cells comparable to skin cells.
The staff, based mostly primarily at Oregon Health and Science University in Portland, devised a way to take away the additional chromosomes by mimicking pure cell division in a means that causes one set of 23 to be discarded, leaving a functional egg cell. The researchers dubbed the method “mitomeiosis.”
However, fewer than 9% of the eggs created in the course of the examine went on to attain the blastocyst stage of embryo improvement, equal to 5 or 6 days postfertilization. This is the time when embryos are often transferred to the uterus throughout in vitro fertilization remedy.
Moreover, Amato defined that each one the ensuing embryos have been chromosomally irregular, both as a result of they’d the unsuitable whole variety of chromosomes or not one from every pair. The embryos wouldn’t be anticipated to end in wholesome infants and certain would all cease creating prematurely, she added.
The examine authors stated intensive further analysis is critical to make the approach protected and environment friendly earlier than it may be utilized in clinics. Specifically, extra examine is required to higher perceive how chromosomes pair and separate to create eggs with the proper variety of chromosomes.
Even in pure copy, solely a few third of embryos develop to blastocyst stage, famous examine coauthor Shoukhrat Mitalipov, director of the OHSU Center for Embryonic Cell and Gene Therapy, in a information launch.
“At this stage it remains just a proof of concept and further research is required to ensure efficacy and safety before future clinical applications,” in accordance to the examine.
Other consultants within the area, comparable to Amander Clark, a professor of molecular, cell and developmental biology at University of California, Los Angeles, are additionally cautiously optimistic. Clark, who was not concerned within the new examine, stated though the analysis is a formidable development, the know-how in its present kind wouldn’t work as a fertility remedy.
“All the embryos were genetically abnormal. Therefore, this approach will not, and should not, be offered in the IVF lab until technical improvements are made,” stated Clark, who can be the director of the UCLA Center for Reproductive Science, Health and Education.
Nonetheless, as a result of hundreds of thousands of ladies endure from main ovarian insufficiency, when their ovaries generate only a few eggs, or their retrieved eggs don’t work in IVF, Clark stated that the strategy reported within the new paper is “an important beginning.”
“To help these women start or build their families transformative medical treatments will be needed as restorative reproductive medicine will not work, and IVF is reaching the limits for treating these types of infertility,” Clark defined in an e mail.
The examine staff made use of somatic cell nuclear switch, a way that was famously used to clone a sheep named Dolly in 1997. In that case, researchers created a replica of 1 mum or dad.
Human reproductive cloning, making a replica of 1 particular person with one set of genetic info, is prohibited in many countries.
The new analysis resulted in embryos with chromosomes contributed from each mother and father. However, as a result of the somatic cell nuclear switch approach is related to cloning, Clark stated “the regulatory barriers for moving this technology into clinical practice will be high.”

The breakthrough is an “exciting proof of concept,” in accordance to Ying Cheong, a professor of reproductive drugs on the University of Southampton and honorary marketing consultant in reproductive drugs and surgical procedure.
“While this is still very early laboratory work, in the future it could transform how we understand infertility and miscarriage, and perhaps one day open the door to creating egg- or sperm-like cells for those who have no other options,” Cheong, who was not concerned within the analysis, stated in feedback shared by the Science Media Centre in London.
“For the first time, scientists have shown that DNA from ordinary body cells can be placed into an egg, activated, and made to halve its chromosomes, mimicking the special steps that normally create eggs and sperm,” she added.